Low-temp hydrocarbon cracking could make plastics from natural gas
In a world that’s hungry for energy and showing no sign of slowing down, there is no industrial process more voracious than petrochemical manufacturing. Since the early 20th century, everything from gasoline and diesel fuel to plastics has been made by cracking complex hydrocarbon molecules found in oil, coal and natural gas with tremendous amounts of heat and pressure.
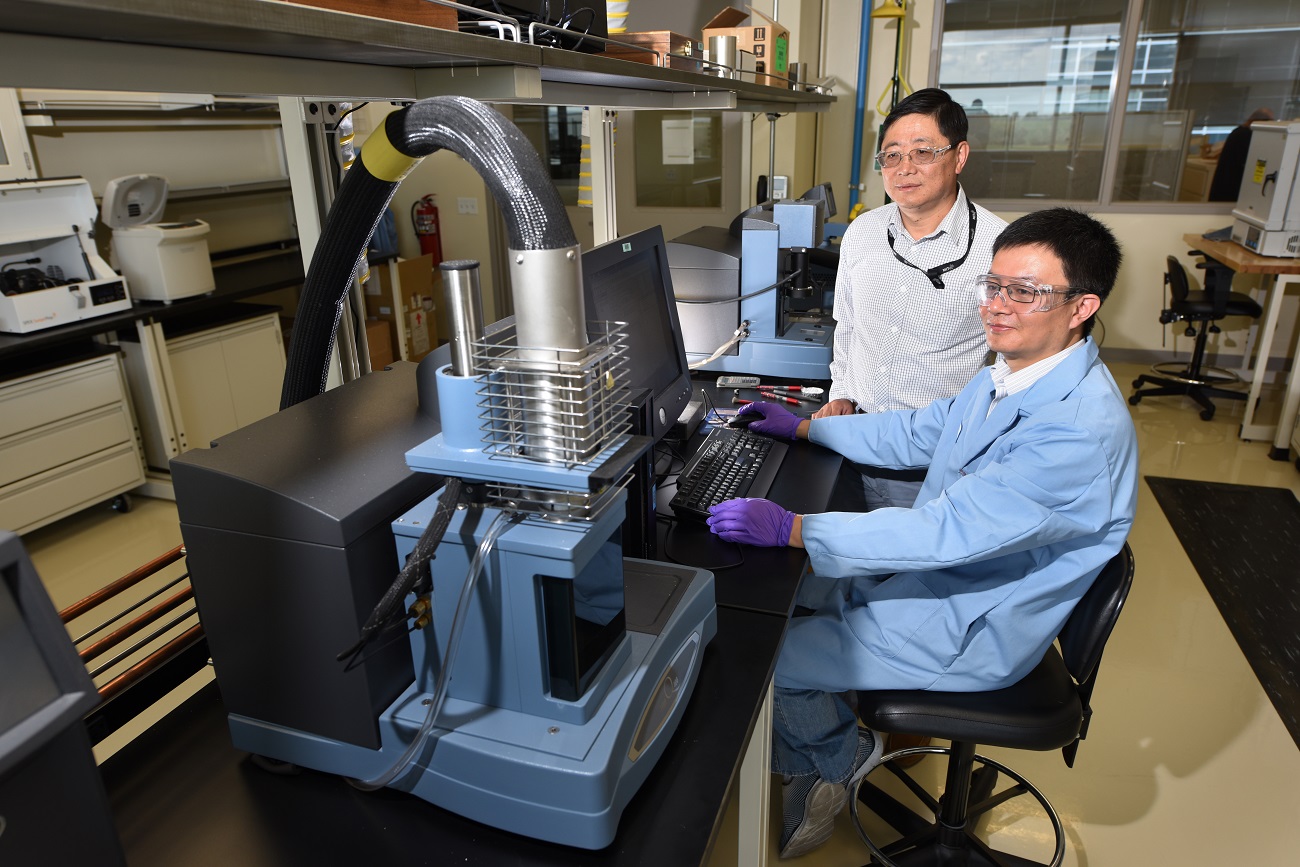
A team of Idaho National Laboratory researchers has now pioneered an electrochemical process that could eliminate the need for high-energy steam cracking. In an article published last week in the scientific journal Energy and Environmental Science, the researchers report they’ve hit upon a process for creating synthetic fuels and plastics that uses 65 percent less energy and produces up to 98 percent less carbon dioxide.
Ethane, a major component of natural gas liquids, offers a simpler hydrocarbon to refine than oil. Once ethane is converted to ethylene, it can be used to make polymers for everything from cellphone cases to disposable diapers.
This conversion can be done thermally, the same way as it is with oil, at temperatures of up to 850 C. But the new process involves feeding ethane to the anode in an electrochemical membrane reactor. Electricity separates protons (hydrogen ions) from the molecules, leaving ethylene, an unsaturated hydrocarbon. Meanwhile the protons migrate through a dense electrolyte to the cathode, where they combine with electrons to form hydrogen gas.
Laboratory-Directed Research and Development (LDRD) funding supported INL’s initial research, which is now being conducted in conjunction with Massachusetts Institute of Technology and the University of Wyoming. The project is one of 24 being funded by the U.S. Department of Energy’s Office of Energy Efficiency and Renewable Energy (EERE). The EERE Advanced Manufacturing Office announced Feb. 5 that the project would receive funding as part of $35 million awarded to 24 projects developing early-stage innovative technology for advanced manufacturing.
Several factors are driving the project, said INL researcher Dr. Dong Ding. First, the shale gas revolution has provided a plentiful supply of natural gas at historically low prices. Second, the declining cost of electricity makes electrochemical refining more economically feasible.
Theoretically, if the process was to be powered by a renewable source and the captured hydrogen was incorporated into fuel cells, there is net gain in process energy. From a CO2 standpoint, using a noncarbon source of electricity — nuclear, hydro, wind or solar — could cut the carbon footprint down to 2 percent of traditional production methods.
Next, the INL team will focus on how to convert methane into ethylene. Methane is also found in natural gas — more plentifully than ethane, in fact — but its carbon-hydrogen bond is much harder to break, Ding said.
Peer reviewers for the Energy & Environmental Science article called the work convincing, timely, original and highly interesting.