Every day, researchers around the world develop new technologies to usher in a carbon-free energy future.
A big part of that effort is developing new materials with advanced properties to make these technologies more flexible, efficient and reliable.
Over the past decade, just as the invention of the silicon microchip revolutionized electronics, crystalline minerals called perovskites have helped researchers discover new, innovative electronics and energy technologies.
Perovskites aren’t exactly new. Discovered in the 1800s, perovskites, which refers to a class of materials with the same crystal structure, have been used in technologies like sensors and lasers for decades.
A gold rush of innovation
More recently, a deeper understanding of perovskite chemistry, structure and properties has led to a sort of gold rush of innovation.
Starting in 1999, researchers discovered that some perovskites could be used to convert light into electricity. This sparked innovations that could soon lead to highly efficient, affordable solar panels.
Now, at Idaho National Laboratory, researchers are using perovskites for different energy applications: converting fuel into electricity or producing valuable chemicals such as ethylene, hydrogen or ammonia.
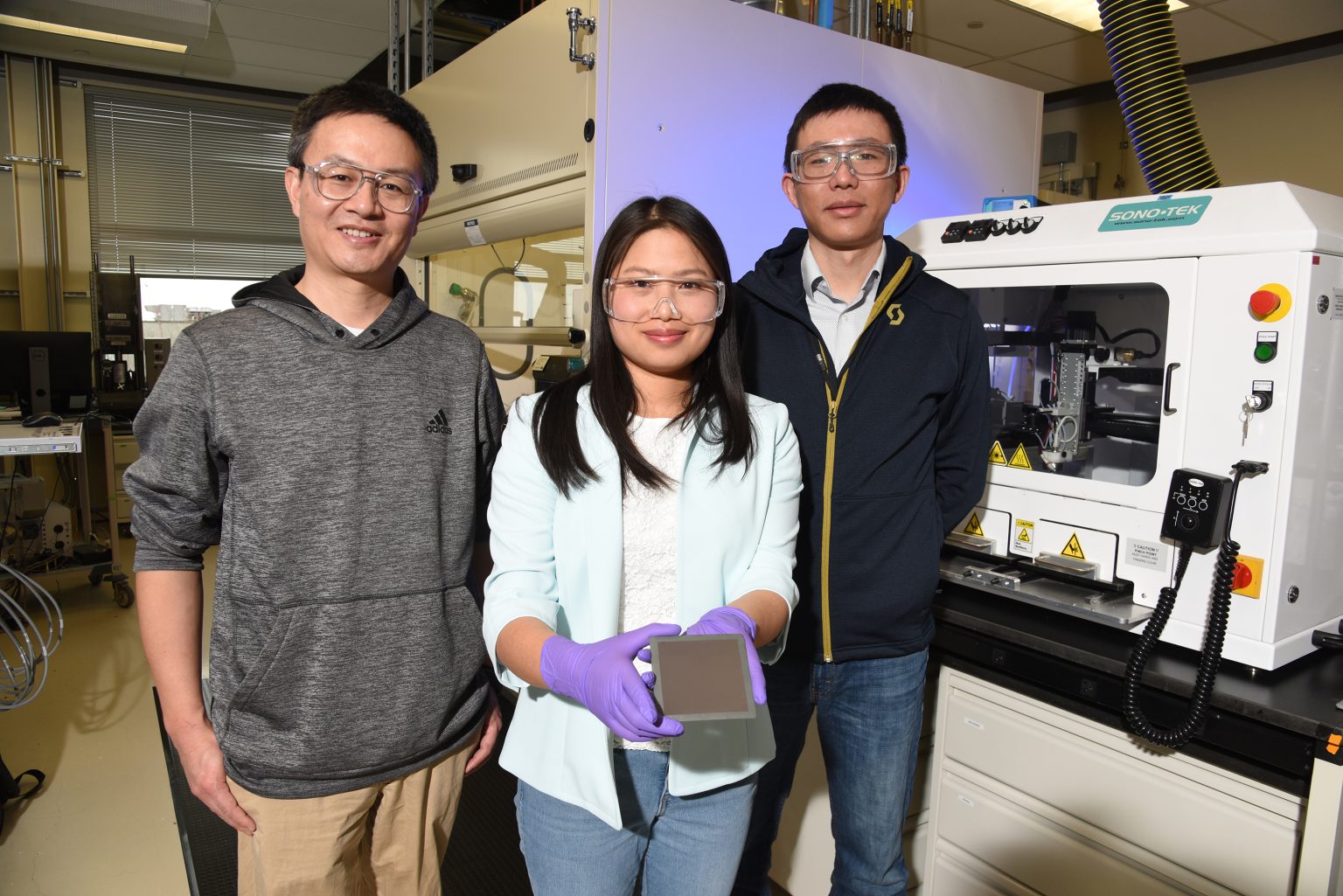
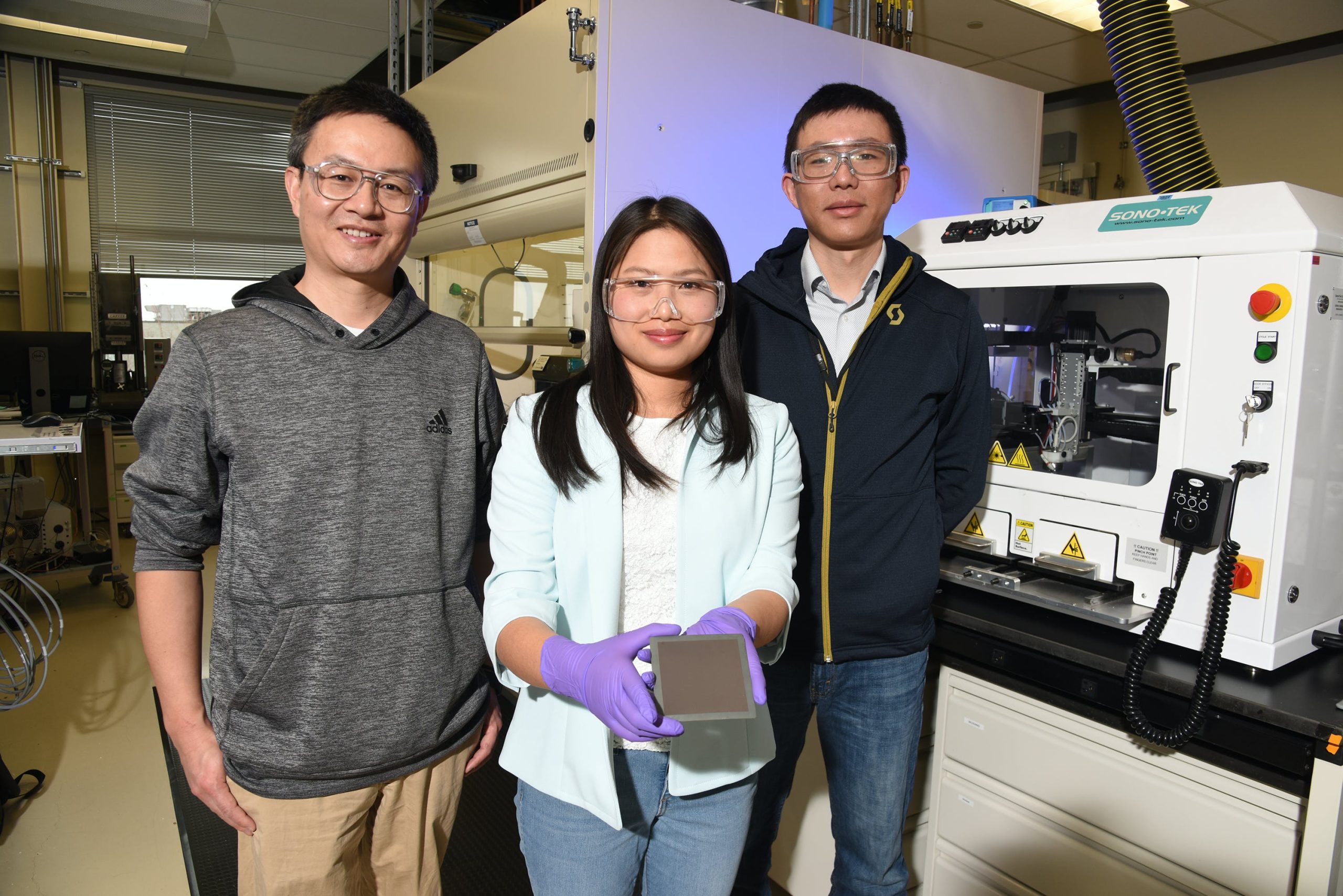
Dong Ding, a distinguished staff engineer/scientist at INL, uses perovskites to construct protonic ceramic electrochemical cells (PCECs). An electrochemical cell is similar to a battery that either generates electricity from a chemical reaction or uses electricity to power a chemical reaction.
Ding and his colleagues have developed PCECs that can convert excess electricity and water into hydrogen, or they can operate in reverse to convert hydrogen into electricity. The electrochemical cells could eventually be used for grid-scale electricity storage. The hydrogen produced by these cells can also be used as fuel for heat, vehicles, chemical production or other applications.
Ding has also used perovskites in an electrochemical cell that readily converts gaseous carbon dioxide into valuable compounds: carbon monoxide or methane. These compounds are important precursors for any number of industrial processes or products, especially syngas, which can be used to reduce U.S. reliance on fossil fuels.
Reducing costs while maintaining performance
Perovskites reduce costs while maintaining the same or similar reaction activity.
This is because both types of electrochemical cells use perovskites in the electrolytes and the electrodes, making the cells both inexpensive and capable of operating at a wide range of temperatures. Previously, these components were made using platinum-based materials.
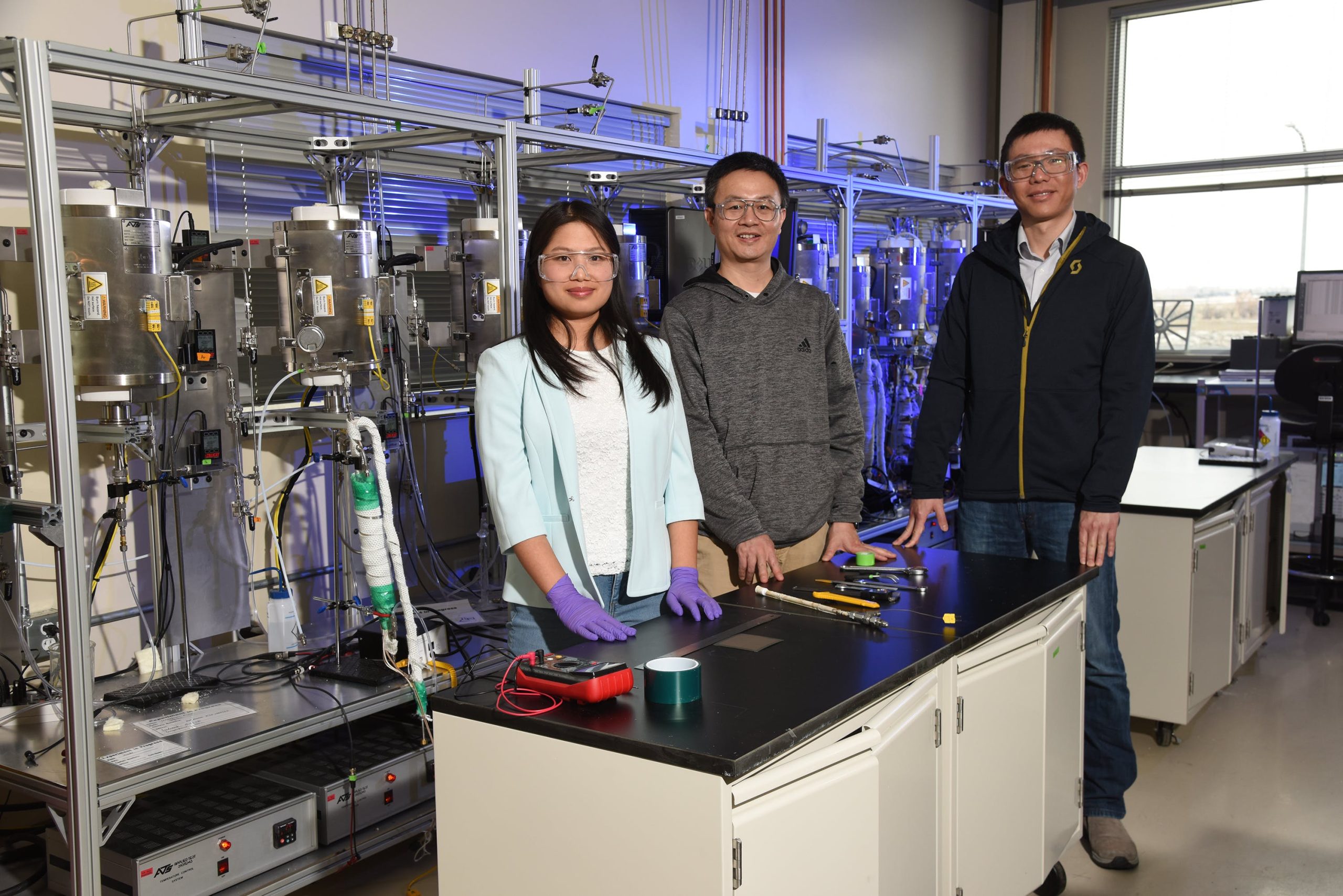
Likewise, Yixiao Wang, a research scientist at INL, uses perovskites in a version of a technology called a membrane reactor. A membrane reactor is a device that uses a membrane to selectively add or remove specific products during a chemical reaction.
Wang and her colleagues use perovskites to facilitate a chemical reaction that converts the methane into ethylene — a valuable chemical that serves as a starting place to synthesize a number of polymers and downstream chemicals. The global ethylene market is expected to reach $231 billion by 2029.
Creating custom perovskites
Perovskites can occur naturally or be made in a laboratory. They come in a variety of chemical arrangements, all based on a similar chemical formula. But their properties and flexibility can vary widely.
In the laboratory, researchers can customize perovskites for certain characteristics.
Two elements, elements A and B, are on the corners of the perovskite. Oxygen atoms lie midway along the sides.
Modify the chemistry by changing A and B atoms and the structure changes, giving a range of different, useful properties.
Replace certain elements with others and the perovskites become better electricity conductors. Perovskites with lanthanum and aluminum can be used to generate lasers at specific wavelengths, for example.
Metal halide perovskites are good semiconductors. Other types of perovskites are magnetic, superconductive, or can create electricity from pressure or light.
Ding’s research group uses perovskites doped with specific elements at the A and B locations to increase the ability of protons (positively charged hydrogen atoms) to flow across the electrode and the electrolyte. This makes the chemical reaction that splits water into hydrogen and oxygen more efficient.
Facilitating different reactions
By replacing the perovskites on the electrode and the electrolyte, Ding and his team could modify their original electrochemical cell designs to perform other types of reactions.
“If you fine-tune your perovskite, it can facilitate many different electrochemical reactions for a wide range of applications,” Ding said. “The way you change the dopants or doping can help formulate useful, high value chemicals. For instance, one type of perovskite might allow you to convert nitrogen into ammonia — a building block for fertilizers and a carbon neutral fuel.”
In Wang’s membrane reactor, using a modified perovskite — strontium lanthanum aluminate (LaSrAlO4) instead of lanthanum aluminate (LaAlO3), a more conventional perovskite — increased the conversion efficiency in the lab by about 60%.
“The modified perovskite leads to the formation of methyl radicals, which couple to form ethylene,” she said. “These oxides have significant potential for use in membrane reactors since they exhibit excellent thermal stability, high ionic/electronic conductivity, good oxygen diffusion properties, and promising catalytic performance due to their tunable compositions.”
In general, the ability to create many different synthetic perovskites or perovskite-like compounds in the laboratory has opened dozens of new avenues for researchers to explore.
“Perovskites give you a lot of options for different elements to dope with,” Ding said. “Their flexibility is their strength.”