Two unique materials that don’t behave like normal solids, liquids or gases could displace toxic chemicals used in today’s metal production processes.
The new approach uses salts that are liquid at room temperature combined with substances that travel like a gas but dissolve materials like a liquid. Together, they could offer a way to convert high-purity rare earth elements (REEs) to metals without using the toxic molten salts required by traditional methods.
High-purity rare earth metals are required to produce magnets, batteries, microelectronics and other components for everything from clean energy technologies to cellphones to missile guidance systems. A stable supply chain for REEs is necessary for the U.S. to be competitive.
That’s why the U.S. Department of Energy recently awarded funding to researchers at Idaho National Laboratory who are developing a low-temperature process that uses less energy and produces less waste. The Critical Materials Institute (CMI), a DOE energy innovation hub established in 2013, is funding the work.
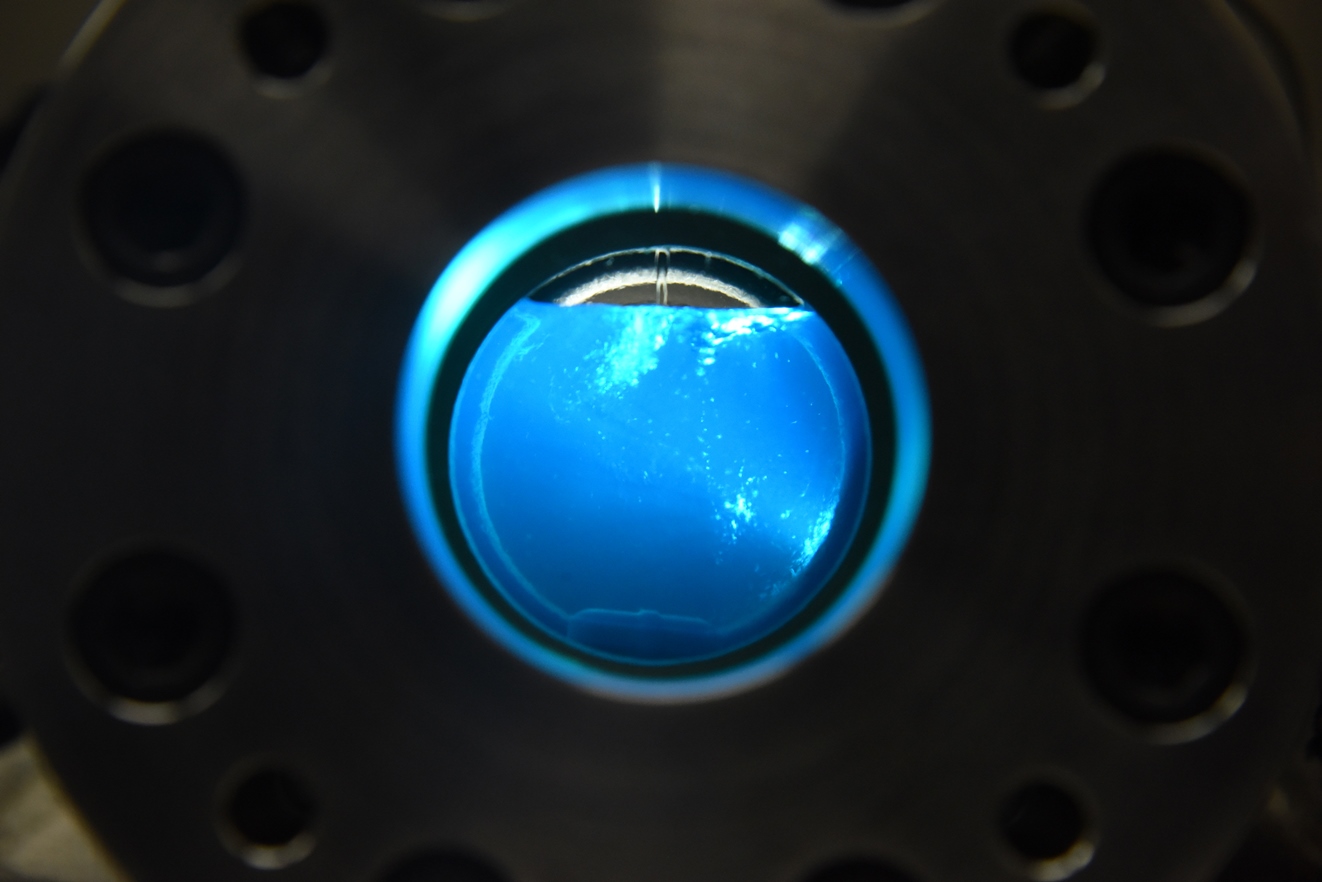
INL chemist Dr. Donna Baek is conducting experiments on how to make metallic forms of REEs using room-temperature ionic liquids (RTILs) and electrochemistry.

RTILs are, as their name implies, salts that are liquid at low temperature. The concept at the heart of Baek’s research is that an electrochemical cell can be used to convert raw powders containing REEs into their reduced metallic form. The key is to dissolve the powders into the RTILs.
The biggest challenge with RTILs is their viscosity, which limits the movement of dissolved ions to the cell’s cathode, where the reduction to pure metal happens. To address this, Baek introduced supercritical fluids, substances kept at a temperature and pressure that allows them to travel through solids like a gas and dissolve materials like a liquid.
Baek was originally focused solely on supercritical fluids. “I pondered for a bit to see how I could get this to work, and that’s when ionic liquids popped into my mind,” she said. “I had previously worked with ionic liquids, so I knew ionic liquids would be the glue I needed to do electrochemistry in a supercritical fluid.”
Rare earth elements are a group of elements found in the Earth’s crust that are not actually all that rare but are hard to separate from each other because they are very similar chemically. Think of a solution containing REEs as a well-mixed spice rub. Once all of the individual ingredients are blended together, it is very difficult to identify the individual components that make up that mixture.
Because REEs are necessary components in cellphones, computer hard drives, electric and hybrid vehicles, and flat-screen monitors and TVs, demand for them has mushroomed. At the same time, production has become more centralized. The U.S. Geological Survey reported that in 1993, the United States produced 33 percent of the world’s REEs. By 2011, offshore sources accounted for more than 97 percent of world production.
The traditional metallurgical method for converting REE salts and oxides to metals was developed in the 1960s by the U.S. Bureau of Mines, said Dr. Robert Fox, a senior chemical researcher at INL who frequently collaborates with Baek. The method involves molten chloride and fluoride salts at high temperatures. The environmental effects and waste streams are extremely toxic.
RTILs are more environmentally benign than high-temperature fluoride and chloride salts, and because the process refreshes the ionic liquid, the waste stream is smaller. The end product is on par with what the molten salt process yields.
“This will be a market disruptive technology for processing and refining REE mixtures from mined and recycled sources,” Fox said. “This technology will help stabilize the REE supply chain by allowing for interconversion between REE oxides and REE metallic forms.”
While results at the lab level have shown promise, the real challenge is scaling up. “Successful chemistry is only the beginning,” Fox said. Researchers have to demonstrate that lab results can be achieved at commercially viable levels, and in the end industry has to embrace a process in order for it to be described as successful.”