Advanced Manufacturing
Advanced sustainable, efficient manufacturing processes
Improved energy efficiency and manufacturing are required for U.S. industries to remain competitive in global markets. INL researchers focus on the challenges of bolstering both energy efficiency and global competitiveness.
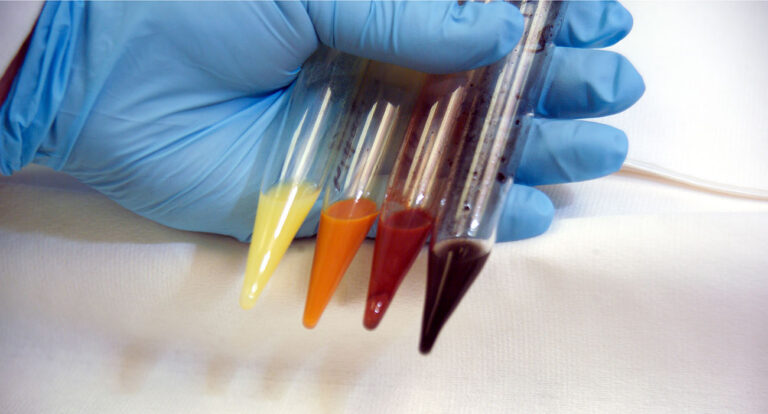
INL is leading research efforts in recycling and reuse of rare and critical materials, which is important to the new Critical Materials Institute. This effort is augmented by developing and testing new specialized materials for manufacturing, as well as the research and application testing of intelligent and resilient systems to produce new, more effective approaches to manufacturing.