At a nondescript laboratory in Idaho Falls, Idaho, Wei Tang, Wenjuan Bian and Dong Ding make highly sophisticated advanced energy materials literally from scratch.
The researchers and their colleagues at the Idaho National Laboratory (INL) are manufacturing an electrochemical energy conversion device called a protonic ceramic fuel cell/protonic ceramic electrolysis cell (PCFC/PCEC). These devices could soon help hydrogen play a big role in the nation’s energy future. The researchers start with powders containing elements and compounds needed for critical materials synthesis, mix them together, and manufacture them into thinner-than-wafer-thin, multilayered sandwiches that form the structure of the ceramic electrochemical devices.
PCFC/PCECs are essentially the same device, just operating in opposite directions. In the fuel cell (PCFC) mode, the device consumes hydrogen in the anode and takes oxygen from air in the cathode to form water and generate electricity. In the electrolysis cell (PCEC) mode, the device converts water and electricity into hydrogen and oxygen.
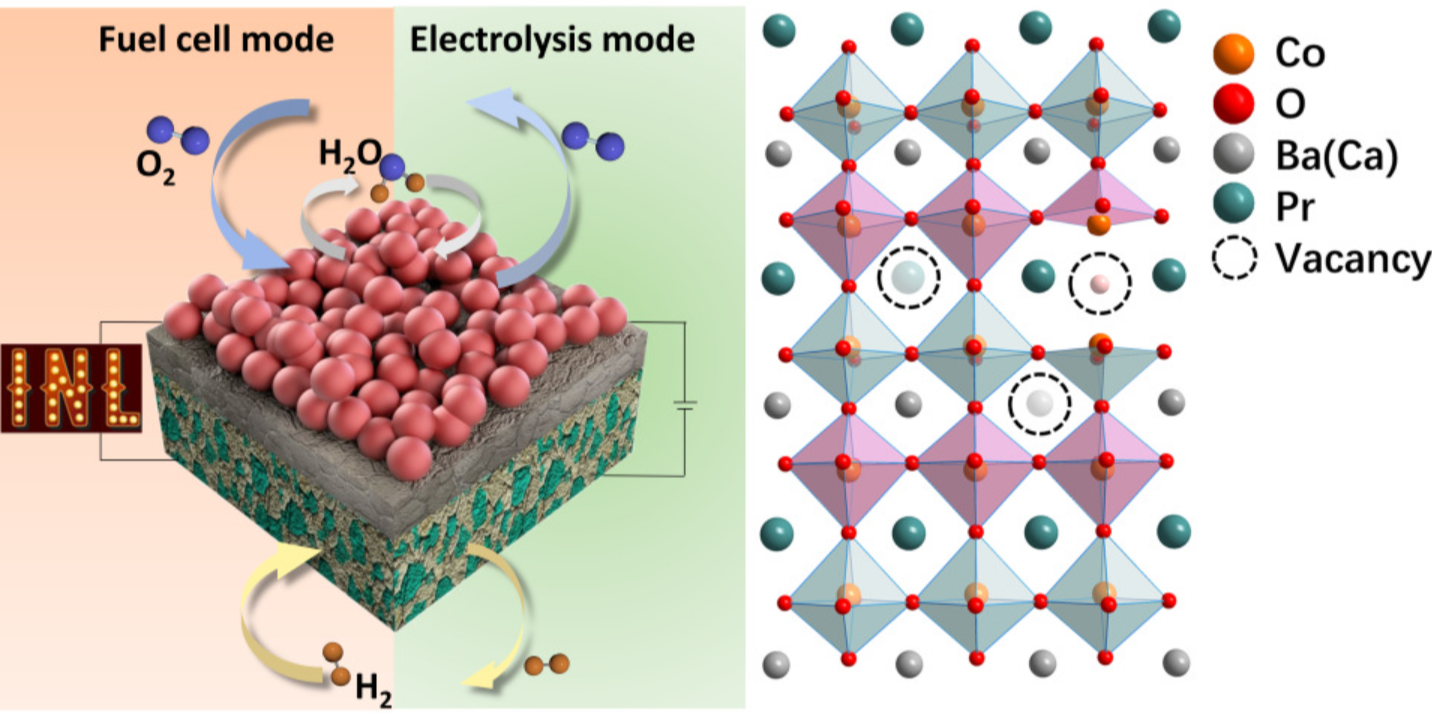
Those two different modes could provide utilities with significant flexibility. When electricity is abundant and its prices are low on the grid, the device can turn steam and electricity into hydrogen, which can be stored or used for industrial chemical production. When electricity is scarce and its prices are high, the cell can operate in reverse and convert stored hydrogen back into electricity.
These devices have another valuable benefit for numerous industrial processes: In contrast to oxygen ion-conducting cells that, in fuel cell mode, generate water molecules on the H2 side or, in electrolysis mode, feed steam on the hydrogen side, PCFC/PCEC devices generate or feed water on the air side. This avoids costly fuel circulation or hydrogen purification steps.
Overcoming key challenges
The researchers have devised a way to manufacture these cells that overcomes several key challenges. The results are published in the highly respected journal Nature Synthesis.
The U.S. Department of Energy’s Hydrogen and Fuel Cell Technologies Office funded the project through the protonic ceramic electrochemical cells program.
To convert inexpensive heat (in the form of steam from power plants, including nuclear plants) and electricity into high-value hydrogen via high temperature steam electrolysis, researchers must make these cells efficient, durable and cost-effective.
While past versions of cells have worked well in the fuel cell direction, (i.e. converting hydrogen into electricity) they’ve become unstable in hydrogen production mode due to the high concentration of steam needed for efficient hydrogen production.
To increase the technology’s stability, the researchers tinkered with the multilayer ceramic sandwich and came up with a solution.
Perovskites: Ceramics with useful, customizable properties
The basic material used to build the PCEC device is a ceramic called perovskite. This family of compounds has a crystalline structure with a high tolerance for composition modifications, allowing for corresponding changes in their physical and chemical properties. Perovskites and their ilk have uses across a wide range of energy technologies, including fuel cells and solar panels.
Researchers have tried to build PCECs using two ceramic layers that are sintered (joined together) together with high temperature heat. However, depending on the type of ceramic used, either the hydrogen production efficiency or long-term durability would suffer.
The state-of-the-art ceramic proton-conducting electrolytes include BaCeO3, BaZrO3 doped with earth elements (i.e. Yttrium, Ytterbium), and their solid-solutions BaZrxCe1-xO3. Cerium-rich perovskite electrolytes generally exhibit higher conductivity, but suffer from poor chemical stability in acidic atmospheres, such as steam. The solution to high-steam-pressure corrosion is using a Ce-free perovskite electrolyte, such as BaZr0.8Y0.2O3−δ (BZY), which is much more stable under high steam conditions during water electrolysis.
Despite being the best candidate for PCEC operation, BZY is difficult to sinter into a dense form. This flaw increases processing costs and negatively impacts the performance of electrochemical cells.
To overcome these challenges, the researchers made a novel structural engineering design. They redesigned the half-cell multilayer structure: they blended high-sinterability electrolyte BaCe0.7Zr0.1Y0.2O3−δ (BCZY) with nickel oxide (BCZY+NiO) for the support layer and used a buffer layer (BZY+NiO) to reduce sintering mismatches between support layer and electrolyte.
These modifications create four layers of the device consisting of: a porous air/steam electrode; a thin perovskite oxide electrolyte made of barium, zirconium and yttrium (BZY); a functional layer made of BZY electrolyte and nickel-oxide (BZY + NiO); and a support layer of barium, cerium, zirconium, yttrium and nickel-oxide (BCZY + NiO).
The high-sintering active support layer assists the densification of the electrolyte at a much lower temperature. The additional functional layer also improves performance by preventing the diffusion of cerium into the electrolyte, which would cause instability during the high steam condition.
Further, the functional layer reduces shrinkage stress of the high sintering active support layer relative to the electrolyte, which also improves the cell’s structural stability at higher temperatures.
Better performance and stability
The resulting electrochemical cell shows better performance in the efficiency with which the electricity provided to the electrolysis cell converts steam into hydrogen (called Faradaic efficiency). In other words, the new cell has a higher hydrogen production rate.
“The material can conduct more ions and results in less electronic leakage for the hydrogen production under a high-steam atmosphere, which is a harsh condition for ceramic electrolyzers” Bian said. “The higher conductivity and longer lifespan here means better performance. Our device shows the best hydrogen production performance. Achieving both excellent performance and long lifespan is typically difficult, but we succeeded through structural design and engineering.”
This material allows more water in the form of steam to be added into the system. “The Faradic efficiency is way higher,” Tang said. “With this high current density, it’s more efficient to generate hydrogen. There is less energy waste, a higher hydrogen production rate and higher energy efficiency.”
Baking the energy future from scratch
Making these highly sophisticated cells is not entirely different from baking in a kitchen. First the ingredients — the precursors of barium, cerium, zirconium, and yttrium — are poured into a mixer. In this case, the mixer is a planetary ball milling machine that mixes the powders and breaks down the particle size for better homogeneity. The mixture is calcinated for ceramic electrolyte material synthesis.
The electrolyte mixed with nickel oxide, and organic solvent and functional additives, are then cast onto a film and baked. The result is a pleasing shade of green film on a semi-transparent sheet of plastic — not too dissimilar from a fruit roll-up snack. The different ceramic films are then placed on top of one another and heated again at 1,450 C — the sintering process that joins the materials together.
The team has successfully scaled up to 25 cm² cells using this technology, a crucial step in bridging the gap between small button cells and a stack of cells that would be used in a full-scale, industrial device. The researchers are now focused on advancing the technology to higher readiness levels.
If their efforts are successful, the device could play an important role in the nation’s energy future where hydrogen is an important chemical precursor, energy storage and transportation medium, and fuel.
“PCEC is definitely a game changer at intermediate temperatures,” said Ding, an INL program manager and Directorate Fellow who has advocated establishing the PCEC program since 2016. “Operating within this temperature range enables the use of cost-efficient materials and can mitigate the rapid degradation typically seen at higher temperatures. This is key to driving costs down and making the technology viable for commercialization. Advanced manufacturing techniques are critical to overcoming the scale-up challenges of PCEC technology, and we are making strong progress at INL”
In addition to producing hydrogen, PCEC can serve as a platform for a wide range of applications for process-intensified chemical and fuel production, such as natural gas upgrading, carbon monoxide conversion and ammonia synthesis by changing the feedstocks and tailoring the catalysts. “We are closely collaborating with the industrial partners at different scales, ” Ding said.